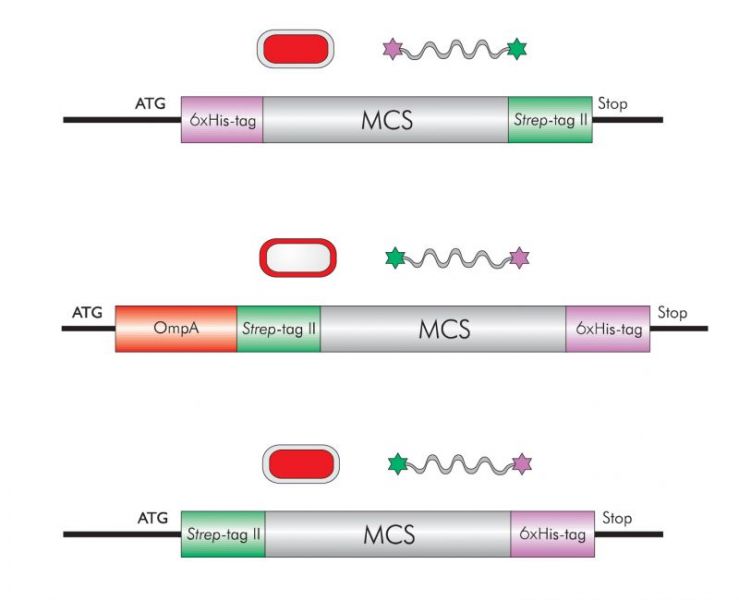
Double-tag proteins
Protein expression is a complex topic with many variables. Therefore, it is e.g. hard to predict whether a recombinant protein is expressed soluble or forms inclusion bodies or is partially degraded. To be prepared for the most common difficulties (see below) the attachment of two tags to a recombinant protein provides the flexibility to obtain a highly pure and homogenous protein preparation.
Purification of full length proteins
Recombinant proteins may be partially degraded during expression which cannot be prevented by adding protease inhibitors during downstream processing. Soluble degradation products still carrying the tag are co-purified and cause an inhomogeneous protein preparation with protein fragments of different lengths. This problem can be solved by adding a second tag to the other protein terminus. Performing a second purification run using the affinity of this second tag selects for full-length proteins.
Highest purification factors
Strep-tagЂч purification enables isolation of recombinant protein at over 99% purity in one step under physiological conditions. However, depending on the recombinant protein, impurities may arise. Although the problem may be solved by changing the resin, the usage of a second affinity tag may potentially be more efficient. To obtain reliable results in any downstream application like, e.g., crystallography, immunizations or HTS, a highly pure product is essential.
Using denaturing OR physiological purification conditions
It is hard to predict whether a recombinant protein is expressed soluble or forms inclusion bodies. This depends e.g. on the strength of the promoter used for expression and on the folding rates of the protein itself. If the protein is soluble and functional, Strep-tagЂч is the best choice because of its mild purification conditions keeping the protein bioactive. If the protein is insoluble and forms inclusion bodies after expression, it has to be solubilized with chaotropic salts like guanidine or urea at high concentrations in order to allow affinity chromatography. Purification under such conditions can be excellently achieved by using the metal chelate activity of the 6xHistidine-tag. Proteins can be purified and optionally be refolded on Ni-NTA columns. If necessary, they can be further purified as refolded protein via Strep-tagЂч in physiological buffer.
Optimizing purification directly from the culture medium
Especially for higher expression hosts (e.g. insect cells) an often applied expression strategy is the secretion of the recombinant protein to the culture medium. In such cases a first capturing step with the 6xHistidine-tag is recommended. Due to the high affinity of the 6xHistidine-tag to Ni-NTA matrix, the recombinant protein can be efficiently collected even by performing batch purification. As a positive side-effect, biotin, which is incompatible with Strep-tagЂч purification and generally present in culture media, is removed in this step. Thus, if necessary, a second purification step can be performed using Strep-tagЂч leading to highly pure protein.
 IDT
IDT