StarGateЂч Transfer Cloning Principle in Detail
Gene transfer into Acceptor Vector
In the transfer reaction step, the GOI may be transferred from the Donor Vector into a variety of Acceptor Vectors providing the desired genetic surroundings (i.e. tag, promoter (Prom), signal sequence etc.) by means of StarCombinase2. The resulting Destination Vector places the GOI under control of the selected promoter (prom) allowing GOI expression in the selected expression host. In addition, a tag (green) is fused to the C-terminal end of the GOI expression product in this example.
Efficient Subcloning using the StarGateЂч System
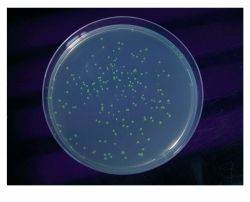
Using StarCombinaseTM the gene for GFP was transferred from a Donor Vector into a pPSG-IBA Acceptor Vector. After an 1 hour incubation, E. coli was transformed with the reaction mixture. All resulting clones exhibited green fluorescence by GFP expression. This provides evidence that the StarGateЂч subcloning reaction had performed accurately and reliably to put the GFP gene under control of the T7 promoter.
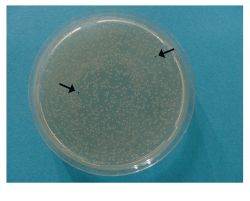
Reaction Efficiency >99%
Transformation of E. coli with a StarGateЂч transfer reaction mixture typically leads to more than thousand white colonies harbouring the desired Destination Vector. The presence of a few blue colonies only indicates a reaction efficiency far beyond 99 %.
Transfer Efficiency is Independent of Gene Size
The green fluorescent protein (GFP, 700 bp), bacterial alkaline phosphatase (BAP, 1400 bp) and T7 RNA polymerase (T7RNAP, 2650 bp) were transferred from the respective Donor Vector into all available Acceptor Vectors (cf. Table, page 4). The result provides evidence that the StarGateЂч subcloning procedure is almost not influenced by the length of the transferred gene and that even large genes can be transferred efficiently.
Transfer efficiency and fragment size
Colony numbers and fragment size
Technical information
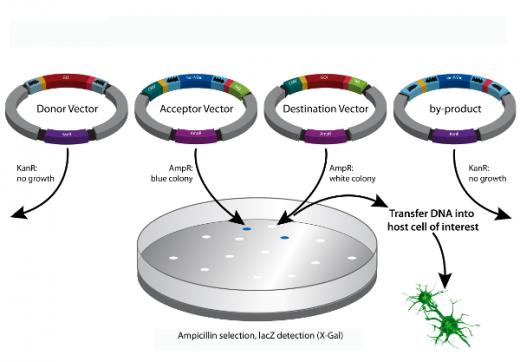
E. coli is transformed with the mixture potentially including all 4 possible vectors. Due to selection on ampicillin plates, Donor Vector and by product – which provide a kanamycin resistance gene only – will not enable growth of E. coli. Acceptor Vector and Destination Vectors, however, enable growth due to the encoded ampicillin resistance genes. The Acceptor Vector carries the LacZa gene and, therefore, produces blue colonies on X-gal containing plates while LacZa has been replaced by GOI in the Destination Vector which, therefore, generates white colonies. The Destination Vector of this example puts GOI under control of the CMV promoter enabling GOI expression in mammalian cells and fuses a tag to the C-terminal end of GOI expression product.
 IDT
IDT