Expression vectors containing double and 6xHistidine tags:
Double-tag and 6xHistindine tags are present in IBA's pASK-IBA and pEXPR-IBA vectors for:
expression of recombinant proteins in E. coli using the tet expression system
and
expression in Mammalia using the CMV promotor providing strong expression in a wide range of mammalian cells.
The choice of system and vector depends on your protein and its characteristics.
Cloning system
Cloning procedure (Classic Cloning)
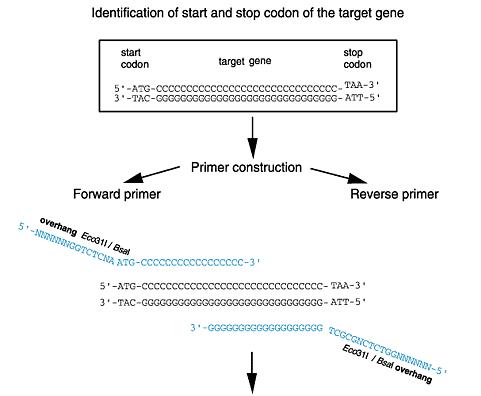
The polylinkers of the expression vectors carry the restriction sites BsaI (isoschizomer Eco31I) and BsmFI (New England Biolabs, MBI Fermentas) which allow the precise fusion of the structural gene with the vector-encoded functional elements (including Strep-tagЂч II, 6xHistidine-tag and, depending on the vector, OmpA-signal sequence, start codon, protease cleavage site or stop codon. This is easily achieved by adapting both ends of the coding region of the structural gene via PCR.
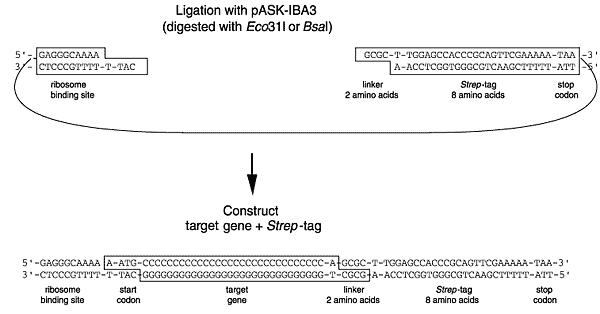
The cloning strategy is described for pASK-IBA3 (see cloning scheme). If a different vector is to be used, the cloning strategy has to be adapted accordingly. The essential primer sequences for each vector are described here or may be deduced by using the Primer D'Signer software (see below).
In cases where other restriction sites are intended to be used for cloning, care must be taken to ensure the in-frame fusion of the structural gene and the vector encoded functional elements.
In the vectors pASK-IBA4 to pASK-IBA7, pASK-IBA35, pASK-IBA37, pASK-IBA44 and pASK-IBA45 with N-terminal affinity tags (see vector overview) the tag is followed by the linker sequence 5'-GGCGCC-3', which is recognized by four different restriction enzymes (KasI, NarI, EheI and BbeI). These four enzymes cut the linker sequence in four different ways. Thus, cleavage with the suitable enzyme and a subsequent filling reaction enable the production of blunt ends in all reading frames in case the target gene insert requires a particular reading frame.
To avoid the incorporation of base substitutions, PCR should be performed with a proof-reading DNA polymerase such as Pfu (Stratagene). 3' phosphorothioate-protected primers should be used in order to avoid 3'5' degradation by the proof-reading activity.
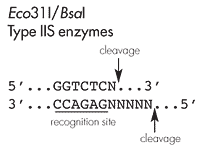
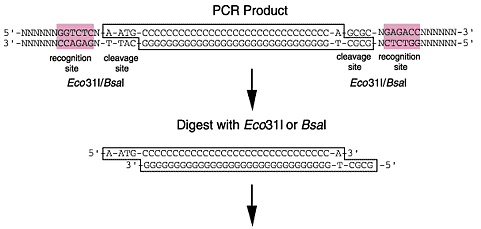
Eco31I and BsaI belong to the Type IIS restriction enzymes which cleave the DNA double strand outside their recognition site. Thereby, the digestion with one single enzyme can generate two different independent sticky ends with 4-base 5'-overhangs allowing directional cloning. In addition, the digestion reaction removes the recognition sequence not affecting the encoded amino acid sequence and expressing authentic protein.
Cloning scheme (demonstrated for pASK-IBA3)
* If a different vector is to be used, the cloning strategy has to be adapted accordingly. In principle the procedure applies to pASK-IBA; pPR-IBA and pEXPR-IBA vectors.
Precise fusion using Eco31I or BsaI
Expression system
Tet promoter
Principle and properties
pASK-IBA vectors work with the tightly regulated tet promoter. The tet repressor is encoded on the pASK-IBA plasmids and is constitutively expressed from the b-lactamase or the chloramphenicol acetyl transferase promoter, respectively. This special arrangement guarantees a balanced stochiometry between repressor molecules and plasmid copy number. Expression of the foreign gene is stringently repressed until efficient chemical induction with a low concentration of anhydrotetracycline. In contrast to the lac promoter - which is leaky, susceptible to catabolite repression (cAMP-level, metabolic state), and influenced by chromosomally encoded repressor molecules - the tetA promoter/operator is tightly controlled and not functionally coupled to any cellular regulation mechanisms or genetic background.
As a consequence, special E. coli strains or extra plasmids are not required and a broad range of culture media and conditions can be used. For example, glucose minimal media and even the XL1-Blue bacterial strain, which carries an episomal copy of the tetracycline resistance gene, can be used for expression. The pASK-IBA expression system is stable under many conditions, including fermentation, and is easy-to-handle.
Further elements of the vectors are a tandem ribosome binding site (RBS) which ensures efficient initiation of translation, the strong terminator of the lipoprotein gene in order to prevent read-through, the intergenic region of the bacteriophage f1 which provides a means for preparing ssDNA and a b-lactamase or chloramphenicol acetyl transferase gene*. The vectors do not mediate resistance against tetracycline.
*Using cloning vectors with b-lactamase resistance gene may be associated with some limitations since ampicillin is degraded quite fast in bacterial culture medium. Therefore, we are now offering our Strep-tagЂч II vectors pASK-IBA2C to pASK-IBA7C with chloramphenicol resistance instead of ampicillin resistance.
Reference:
Skerra, A. (1994). Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli. Gene, 151, 131-135.
The following E. coli strains have already been used successfully for Tet expression with our pASK-IBA vectors: JM83, WK6, B, BL21, MG1655, W3110, BL21(DE3), BLR(DE3), XL1-Blue, BL21-CodonPlusTM-RIL
For secretion, we recommend JM83. For cytoplasmic expression E. coli B strains are recommended, since they lack the lon protease and the ompT outer membrane protease that can degrade proteins during purification (Grodberg and Dunn, 1988, J.Bacteriol. 170, 1245).
Please note that we are not aware of an E. coli strain that is incompatible with the Tet expression system.
Anhydrotetracycline: Inducer for tetA Promoter
The E. coli expression cassette of the Strep-tagЂч/Strep-TactinЂч over-expression system is under transcriptional control of the tetA promoter/operator and repressor. The promoter is induced by a low concentration of anhydrotetracycline (AHT) saving costs and minimizing the antibacterial influence of AHT. Degenkolb et al. (1991) have shown, that AHT binds 35-times tighter than tetracycline to the tet repressor.
Comments on E. coli expression
Formation of disulfide bonds
Some vectors provide a N-terminal fusion of the ompA signal peptide which mediates the secretion of the recombinant protein to the periplasmic space of E. coli. There, the signal peptide is selectively cleaved by the E. coli signal peptidase. The secretion strategy is essential for the functional production of proteins containing structural disulfide bonds that are often present in naturally secreted proteins. The reducing conditions in the cytoplasm of E. coli prevent disulfide bond formation which can lead to aggregation or degradation of unfolded polypeptides.
Periplasmic secretion as a first purification step
Furthermore, periplasmic secretion separates the recombinant protein from cytosolic proteases. Since the E. coli outer membrane can be selectively degraded by mild treatment (EDTA, lysozyme etc.) the spheroplasts containing the cytosolic components can be easily removed by centrifugation.
Addition of active substances
In addition, the periplasmic space is accessible to molecules < 600 Da allowing to influence folding or stability of the recombinant protein during expression by adding active substances to the culture media (e.g. redox components, non-metabolizable sugars, ligands of the recombinant protein etc.).
Cytoplasmic or periplasmic expression
As long as a cytoplasmic recombinant protein does not include stop-transfer sequences, the advantages of periplasmic secretion are also amenable to this type of protein. However, because stop-transfer sequences are difficult to predict, it is advisable to try both strategies in parallel. Using the vectors for N-terminal Strep-tagЂч II or 6xHistidine-tag fusion, the change from cytoplasmic to periplasmic expression (and vice versa) can be achieved by a simple cloning step via the NheI/BstBI and the EcoRV/HindIII restriction sites on the 5'- and 3'- end, respectively.
Do you need an authentic protein?
For special applications requiring an authentic protein, several vectors encoding the factor Xa protease cleavage site adjacent to Strep-tagЂч II/6xHistidine-tag are available allowing the complete removal of the tag.
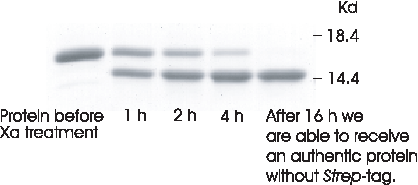
Factor Xa processing
Protein gel demonstrating the treatment of the 15 kDa selenoprotein (carrying a N-terminal Strep-tagЂч) with factor Xa (1:1000, w/w) at 22ЁЦC. The gene encoding the protein had been cloned into pASK-IBA6, which contains a Xa cleavage site adjacent to Strep-tagЂч II.
Lane 1, protein before Xa processing; lane 2, after 1 hour incubation; lane 3, after 2 hours; lane 4, after 4 hours; lane 5, after 16 hours: homogenous authentic protein without Strep-tag II.
Strains
The following E. coli strains have already been used successfully for Tet expression with our pASK-IBA vectors: JM83, WK6, B, BL21, MG1655, W3110, BL21(DE3), BLR(DE3), XL1-Blue, BL21-CodonPlusTM-RIL
For secretion, we recommend JM83. For cytoplasmic expression E. coli B strains are recommended, since they lack the lon protease and the ompT outer membrane protease that can degrade proteins during purification (Grodberg and Dunn, 1988, J.Bacteriol. 170, 1245).
Please note that we are not aware of an E. coli strain that is incompatible with the Tet expression system.
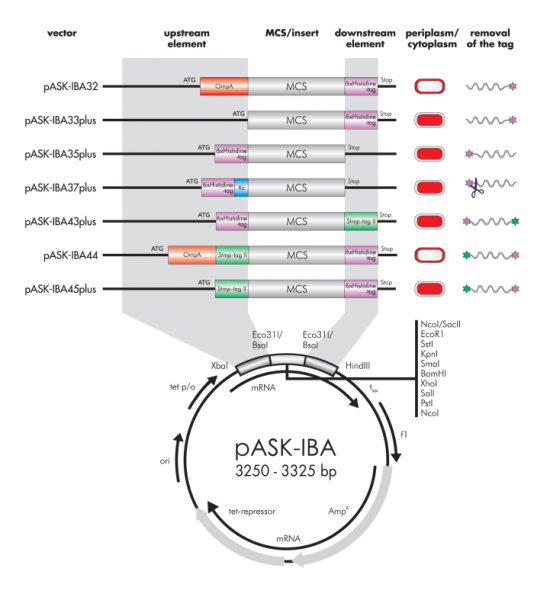
6xHistidine and double tag Vector features
In addition to Strep-tagЂч vectors, we are also offering double-tag vectors with both, Strep-tagЂч and 6xHistidine-tag, as well as 6xHistidine-tag vectors. Two different affinity tags on a protein yield highest purification factors and allow purification of full-length proteins under denaturing or physiological conditions. Also, purification protocols directly from the culture medium can be optimized.
6xHistidine-tag and double-tag vectors
Sequencing primers
|
Product |
amount |
cat. no. |
|
Forward sequencing primer for
pASK-IBA vectors |
1 nmol, 10 pmol/µl
HPLC purified |
5-0000-101 |
|
Reverse sequencing primer for
pASK-IBA vectors |
1 nmol, 10 pmol/µl
HPLC purified |
5-0000-102 |
|
Forward and reverse sequencing primers for pASK-IBA vectors |
1 nmol each, 10 pmol/µl HPLC purified |
5-0000-104 |
References
Tet promoter
Tet promoter:
- Skerra, A. (1994).
Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli.
Gene 151, 131-135.
- Degenkolb J, Takahashi M, Ellestad GA, Hillen W. (1991)
Structural requirements of tetracycline-Tet repressor interaction: Determination of equilibrium binding constants for tetracycline analogues with the Tet repressor.
Antimicrob. Agents Chemother. 35 (8), 1591-1595
pASK-Vectors
pASK-Vectors:
- Müller I.B., Wu F., Bergmann B., Knöckel J., Walter R.D. (2009) Poisoning Pyridoxal 5-Phosphate-Dependent Enzymes: A New Strategy to Target the Malaria Parasite Plasmodium falciparum.PLoS ONE 4(2), e440.
- Han, R., Zwiefka, A., Caswell, C.C., Xu, Y., Keene, D.R., Lukomska E., Zhao, Z., Höök, M., Lukomski, S. (2006) Assessment of prokaryotic collagen-like sequences derived from streptococcal Scl1 and Scl2 proteins as a source of recombinant GXY polymers. Appl. Microbiol. Biotechnol. 72, 109–115.
- Humtsoe, J.O., Kim, J.K., Xu, Y., Keene, D.R., Höök, M., Lukomski, S. and Wary, K.K. (2005)
A streptococcal collagen-like protein interacts with the integrin and induces intracellular signalling.
JBC 280, 13848-13857.
pPR-IBA vectors
pPR-IBA vectors:
- Eren E. Kennedy D.C., Maroney M. J., and Argüello J.M. (2006)
A Novel Regulatory Metal Binding Domain Is Present in the C Terminus of Arabidopsis Zn2 -ATPase HMA2,
JBC281 (45), 33881–33891
|
- ЛчПы ШФБт
- ЛчПыШФБтАЁ ОјНРДЯДй.
ЛчПыШФБт РлМК
|
 IDT
IDT