* 2019 РЬШФ УыБо ОШЧд*
ExpressoЂч CMV Cloning and Expression System
- Fast: 5-second, directional cloning with no enzyme incubations, ligation, or vector prep. Watch video »
- Precise: "No scar" vector results in highly accurate sequences and correct proteins.
- Small Vector: 3.4-kb vector gives high transfection efficiency & easy downstream manipulation.
- Robust: CMV promoter provides strong, constitutive eukaryotic expression.
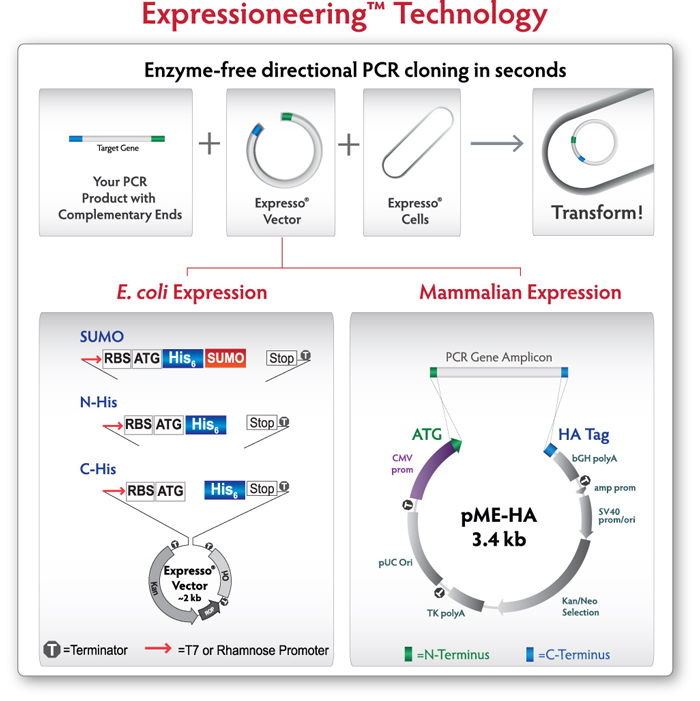
The ExpressoЂч CMV Cloning and Expression System combines instant, enzyme-free, directional cloning with a ЁАno scarЁБ vector for eukaryotic gene expression. This cloning system is based on ExpressioneeringЂт Technology, which uses in vivo homologous recombination to seamlessly clone PCR-amplified DNA into specially designed expression vectors without purification or enzymatic incubations. ItЁЏs the fastest, easiest mammalian expression system available.
Figure 1. Expressioneering Technology uses in vivo homologous recombination to seamlessly clone PCR-amplified DNA into the pME-HA vector. Target genes are amplified with primers that include 18 bases of overlap with the ends of the Expresso vector. The unpurified PCR amplicon is simply mixed with the pME-HA expression plasmid and immediately transformed into the highly competent cells provided. No DNA purification, enzyme treatment, vector preparation, or ligation steps are required.
pME-HA Vector
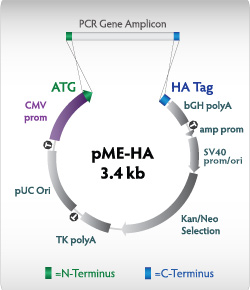
The Expresso CMV Cloning and Expression System features the pME-HA expression vector, which contains all the elements you need, and nothing you don't. The CMV promoter enables strong, constitutive expression in the smallest mammalian expression vector available. The 3.4kb vector supports higher tranfection efficiency and easier downstream manipulation. Combined with Expressioneering technology for instant, scar-free cloning, the result is surprisingly simple mammalian expression.
Figure 2.pME-HA expression vector The CMV promoter drives expression of the PCR insert; amp and SV40 promoters express the gene for kanamycin/neomycin resistance in bacteria and mammalian cells. The pUC origin gives high plasmid yields. CloneSmartЂч transcription terminators (T) prevent transcription into or out of the vector backbone, increasing clone stability. The HA affinity tag can be fused to the carboxy terminus of the expressed protein, if desired.
Strong Expression
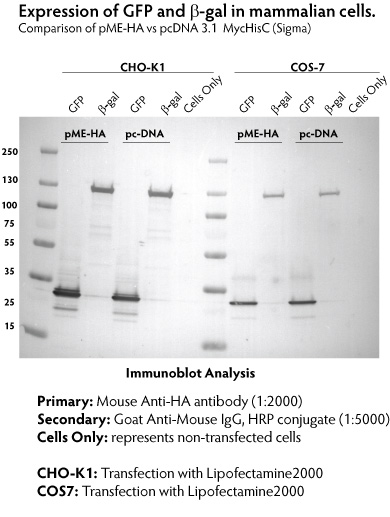
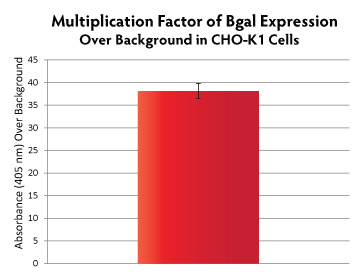
The pME-HA vector provides robust protein expression, greater than or equal to that of the commonly-used vector pcDNA 3.1. Protein expression from the GFP gene (0.8 kb) and the full length Ѕт-galactosidase gene (3 kb) was detected by Western blotting against the HA tag in CHO-K1 and COS-7 transfectants (Figure 3). Ѕт-galactosidase expression was also measured quantitatively in transfected CHO-K1 cells (Figure 4).
Figure 3. Comparison of protein expression from pME-HA and pcDNA3.1 vectors in CHO and COS cells. CHO and COS cells were transfected with constructs encoding Green Fluorescent Protein (GFP) and beta-galactosidase (Ѕт-gal) using Lipofectamine2000 (Invitrogen) following the manufacturer's recommendation. At 48 h post transfection, whole cell lysates were fractionated by SDS PAGE, transferred to a PVDF membrane, and detected with anti-HA antibody. Expression from the pME-HA vector was equal to or greater than that from the ЁАstandardЁБ pcDNA3.1 vector.
Figure 4. CHO-K1 cells were transfected with pME-HA-Bgal plasmid at a ratio of 2:1 (µl TransIT-2020 transfection reagent : µg DNA). Cells were assayed for Bgal activity 24-hours post-transfection using the Mammalian B-Galactosidase Assay Kit (ThermoScientific, # 75707), and read at 405 nm on a plate reader. Absorbance values were divided by background absorbance (mock-transfected cells).
Expression of Difficult Proteins
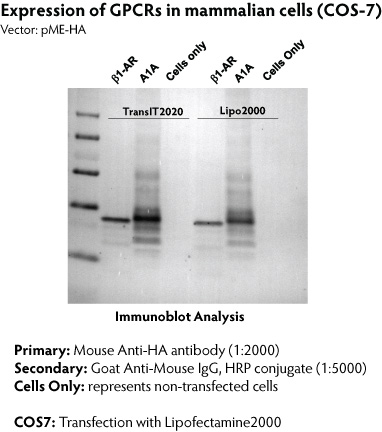
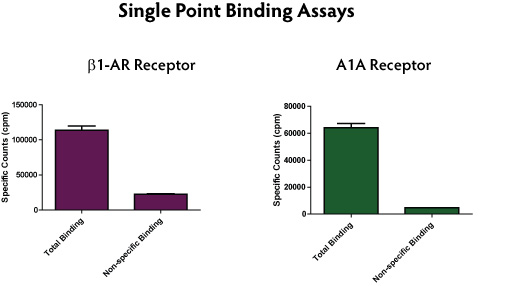
The use of Expressioneering technology results in precise cloning, without the addition of unwanted sequence (such as restriction sites) typically present in multiple cloning sites. The minimal size of the pME-HA vector also contributes to enhanced cloning capability. Numerous GPCR genes have been cloned and expressed in mammalian cells using the Expresso CMV system. Western blotting was used to detect expression of the HA-tagged GPCRs (Figure 5), and functionality was confirmed by radioactive ligand binding assays (Figure 6).
Figure 5. Expression of GPCRs from the pME-HA vector. COS-7 cells were transfected with constructs encoding turkey beta1-adrenergic receptor (Ѕт1-AR) and human adenosine A1 receptor (A1A) with TransIT-2020 (Mirus Bio LLC) or Lipofectamine2000 (Invitrogen) following manufacturer's recommendation. At 48 hours post-transfection, whole cell lysates were fractionated by SDS-PAGE, transferred onto PVDF membrane, and detected with anti-HA antibody.
Figure 6. Radioligand binding data for GPCRs expressed from the pME-HA vector.Cells expressing b1-AR were analyzed for radioligand binding using 20 nM [3H]-Dihydroalprenolol (PerkinElmer). Non-specific binding was determined in presence of excess unlabeled competitor, 10 ЅьM (S)-(-)-Propanolol hydrochloride. Receptor bound ligand was captured on GF/B filters and counted. Cells expressing A1A receptor were analyzed by binding of 10 ЅьM [3H]-DPCPX (PerkinElmer). Non-specific binding was determined in presence of excess unlabeled competitor, 10 ЅьM 8-Cyclopentyl-1,3-dipropylxanthine. Bound ligand was captured on GF/B filters and counted.
 IDT
IDT